| Shukla Das*, Rumpa Saha*, Sambit Nath Bhattacharya**, Kiran Mishra†, Sajad Ahmad Dar*
*Department of Microbiology, **Department of Dermatology and STD, †Department of Pathology, University College of Medical Sciences (University of Delhi) & Guru Teg Bahadur Hospital, Delhi - 110095. INDIA
Corresponding Author: Dr. Shukla Das, Professor, Department of Microbiology, University College of Medical Sciences & GTB Hospital, Delhi - 110095. INDIA E-mail: shukladas_123@yahoo.com
Abstract
Subcutaneous mycoses include a wide spectrum of infections, caused by a heterogeneous group of fungi, of which hyalohyphomycosis is emerging as significant infection in immunocompromised and immunocompetent patients. Acremonium like other hyaline fungi can lead to superficial or deep infection and may even prove fatal. Such infections are rare and treatment outcome may vary depending on the extent of infection and species involved. We report here a case of subcutaneous nodule caused by Acremonium kiliense in a diabetic patient. The infection resolved with fluconazole and terbinafine after eighteen months.
Key words: Fungal infections, Acremonium, Anti-fungal agents. |
Introduction
Many filamentous fungi are common environmental saprophytes that cause a variety of infections mostly thought to be secondary to increased host susceptibility and prior colonization [ 1, 2]. Acremonium, isolated from dead plant material and soil, includes approximately 150 species. Mostly an opportunistic pathogen, this fungus can cause infections like kerion, onychomycosis, keratitis and mycetomas in immunocompetent individuals. Occurrences of disseminated and invasive infections like pneumonia, arthritis, osteomyelitis, endocarditis, meningitis and sepsis in the immunodeficient patients have also been reported. Optimum therapy of Acremonium infection is unclear because of limited documented reports and conflicting results of therapies. Amphotericin B in addition to ketoconazole or fluconazole has been the preferred therapy [ 3]. A case of subcutaneous infection caused by Acremonium kiliense in an immunocompetent patient is being presented along with an analysis of the documented reports of Acremonium infection worldwide and their antifungal susceptibility pattern.
Report of a Case
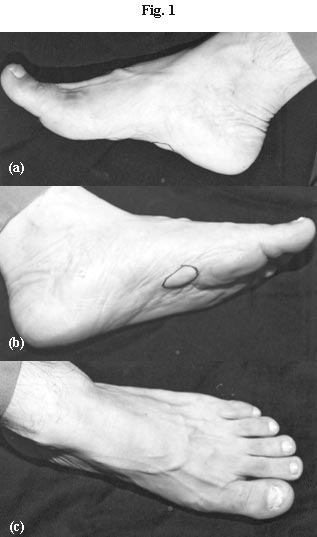
A 46 year old male, presented with six month duration of painless swellings on both the soles. The patient was a lawyer by profession with a habit of wearing closed footwear. There were two distinct lesions on the left sole which appeared earlier, present on the medial aspect, well circumscribed, hard, 3cm by 2cm in size, with intact skin. A similar swelling was present on the right sole of 3cm by 3.5cm in size, also on the medial aspect. There were no sinus tracts or grains. There had been an apparent increase in the size of the swellings, up to 2 cm in diameter in a span of eight months. There was also involvement of the left big toe nail as noted by the patient before attending the out-patient department of Skin and Venereal Diseases of Guru Teg Bahadur Hospital, Delhi, India (Fig. 1a, 1b, and 1c). He had no history of travel in the recent past nor did he recall any history of trauma. He was a known diabetic (Type 2) on insulin but treatment had been irregular and infrequent. He had been prescribed a course of antibiotic by a local practitioner with a probable diagnosis of pyoderma but without significant benefit.
 |
| |
| Figure 1a: Subcutaneous swellings of the right sole. Two swellings on the medial aspect of the sole, well circumscribed, hard, 3cm X 2cm in size, with intact skin. |
Figure 1b: Single swelling on the on the medial aspect left sole of 3cm X 3.5cm in size. |
Figure 1c: Nail involvement of the left great toe showing white superficial onychomycosis. |
|
Blood sugar as recorded during his first visit to the hospital was 300 mg/dL. Other routine laboratory investigations, which included complete blood count (8,000/mm3), urine analysis, X-ray chest were within normal limits. Patient was seronegative for HIV. Routine bacterial cultures were negative. Skin biopsies from both the right and left nodular swelling on the sole were taken for fungal and mycobacterial cultures. Nail scrapings were also taken for fungal workup. Fine Needle Aspiration Cytology (FNAC) was also done from both feet and the fluid sent for analysis. An MRI scan of both the swellings (right and left sole) showed well defined soft tissue lesions in subcutaneous part of plantar aspect of the right and left foot with hypo intense signal on T1W images with hyper intense signal on T2W and STIR images. The lesions were approximately 2.5cm by 2cm and 3cm by 3cm in size. There was no significant fluid in the ankle joint. There was no destruction or discontinuity of integrity of any major ligaments. No sign of any inflammation was observed.
The isolation and identification of the causative agent was performed in the Microbiology laboratory of University College of Medical Sciences & Guru Teg Bahadur Hospital. The KOH (10% and 40%) examination of the biopsy specimens and nail scrapings demonstrated delicate thin hyaline and septate hyphae. No pigmentation or any yeast like cells suggestive of sclerotic bodies was seen (Figure 2a). FNAC examination showed intense acute inflammatory infiltrate with pus cells, numerous foreign body giant cells and eosinophils. On May Grunwald Giemsa (MGG) stain, images of fungi with branching and septate hyphae were seen in the background (Figure 2b). Periodic Acid Schiff (PAS) staining confirmed a similar finding of septate hyphae along with numerous giant cells (Figure 2c).

Figure 2a: 10% KOH mount of biopsy of the subcutaneous swellings (400X Magnification). Delicate thin hyaline and septate hyphae seen in the figure.
Figure 2b: May Grunwald Giemsa (MGG) stain of FNAC material (400X Magnification). Figure shows images of fungi with branching and septate hyphae.
Figure 2c: Periodic Acid Schiff (PAS) staining of FNAC material (200X Magnification). Figure shows multiple giant cells and fungal hyphae. Inset shows septate fungal hyphae in PAS stain (400X Magnification).
Fungal cultures of both biopsy and nail scrapings were performed on Sabouraud’s dextrose agar (SDA) and incubated at 250C. White powdery suede like colonies with a salmon colored base were observed within a week of incubation. LPCB preparation from the cultured colonies revealed septate hyphae bearing right angled branches. Phialides were awl shaped having one celled ellipsoidal conidia with rounded edges accumulating as heads or in chains. The isolate was identified as Acremonium kiliense. The isolate was deposited to the Department of Medical Microbiology, Centre of Advanced Research in Medical Mycology, Post Graduate Institute of Medical Education and Research, Chandigarh, India, for confirmation and antifungal susceptibility testing. Antifungal susceptibility (MIC determination), as per Clinical and Laboratory Standards Institute (CLSI/NCCLS) guidelines [4], showed sensitivity to amphotericin B (0.25 μg/mL), fluconazole (>64μg/mL), itraconazole (>16 μg/mL), Voriconazole (6.5g/ μg/mL) and terbinafine (0.0102 μg/mL). Normoglycemia was achieved by regulating the dose of insulin and subsequently, the patient was started on antifungals. As there was no systemic involvement, the patient received a course of terbinafine 250 mg daily and fluconazole 400 mg weekly. There was a gradual reduction in the size of the lesion and it completely healed after a total of eighteen months therapy. The nodular skin lesions healed with fibrosis and repeat microscopic examination and culture of the toe nail was negative for any mycological evidence. The patient was followed up for a period of two years without any recurrences.
Discussion
Hyalohyphomycosis is an unusual opportunistic mycotic infection. The term Hyalohyphomycosis is specially designated for infections caused by ubiquitous saprophytes such as Fusarium, Scedosporium, Scopulariopsis, Penicillium marneffei, Acremonium etc., which appear as hyaline, septate mycelial elements, with branched or intertwined hyphae without any pigmentation in the cell wall. Fungi belonging to the genus Acremonium are ubiquitous environmental contaminants and soil saprophytes but are infrequent pathogens in humans [5]. The genus Acremonium (also known as Cephalosporium) isolated from dead plant material and soil includes approximately 150 species. Of the 150 known species, nine are implicated as human pathogens - falciforme (currently known as Fusarium falciforme), A. recifei, A. kiliense, A. potronii, A. roseogriseum, A. strictum, A. alabmensis, A. blochi, A. astrogriseum). They differ from genus Aspergillus which have septate hyaline but relatively broad coarse hyphae with characteristic vesicles bearing conidia in one or two rows of phialides. These fungi cause a variety of infections mostly thought to be secondary to prior colonization and increased host susceptibility
Infection has been described in immunocompromised patients; however its invasive potential in immunocompetent patients has been reported rarely. In immunocompromised host it is generally associated with pulmonary or disseminated infections after inhalation or may also arise from direct inoculation. Most infections have presented as mycetoma or ocular infections occurring mainly due to penetrating injury [3,6,7]. In immunocompetent patients, mycetomas due to Acremonium species account for one third cases of the several causes of mycetomas reported worldwide [6]. Reports suggest that among the immunocompromised patients, subcutaneous lesions mostly occur in renal or diabetic patients [8]. Table 1 depicts a worldwide distribution of mycetomas due to Acremonium species.
Table 1: Worldwide subcutaneous infections caused by different species of Acremonium.
Type of Infection |
Species |
Place |
Year |
Treatment |
Outcome |
References |
Mycetoma |
Acremonium spp. |
Brazil |
2008 |
Surgery, Itra & Keto, New azoles |
Not known |
[9] |
Mycetoma in heart transplant case |
Acremonium spp. |
USA |
2006 |
Not Known |
Not known |
[10] |
Mycetoma |
A. kiliense
A. recifei
A. falciforme |
Buenos Aires |
2006 |
Keto, Itra, Clotri |
Cured |
[11] |
Mycetoma |
Acremonium spp. |
Argentina |
2005 |
Not known |
Cured |
[12] |
Subcutaneous
hyalohyphomycosis on cheek |
A. strictum |
Turkey |
2001 |
AmB |
Not cured |
[1] |
Podalic Mycetoma |
A. kiliense |
Brazil |
1999 |
Itra |
Cured |
[13] |
Mycetoma |
A. recifei |
Brazil |
1995 |
Itra |
Cured |
[14] |
Mycetoma |
A. kiliense,
A. falciforme |
Madras |
1995 |
Not Known |
Cured |
[15] |
Subcutaneous leg abscess in renal transplant |
A. falciforme |
Brazil |
1994 |
Surgery, Keto |
Cured |
[16] |
Mycetoma |
A. kiliense |
Hungary |
1991 |
Surgery, Itra |
Cured |
[17] |
Mycetoma |
A. strictum |
France |
1988 |
Flu, Nystatin, AmB |
Cured |
[18] |
Mycetoma |
A. recifei |
South India |
1979 |
Surgery, Keto |
Cured |
[19] |
Mycetoma |
Acremonium spp. |
Madras |
1977 |
Itra |
Cured |
[20] |
Mycetoma |
A. falciforme |
California |
1976 |
Not known |
Cured |
[21] |
Madura foot |
Cephalosporium madurae |
South India |
1966 |
Not known |
Cured |
[22] |
|
| AmB: Amphotericin B |
Keto: Ketokonazole |
Itra: Itraconazole |
| Flu: Fluconazole |
Clotri: Clotrimazole |
|
However, in India, infections with Acremonium are not as frequently reported as other fungal infections; nor is it a well recognized aetiologic agent in causing subcutaneous infections. Pathogenicity of subcutaneous infections with Acremonium species involves granuloma formation with or without development of sinus tract. Sometimes there may also be an underlying predisposing immunological deficit. Unusual presentation of mycetomas caused by Acremonium as in the present case, has limited documentations from India [15,19,20,22]. This rarity of Acremonium mycetoma can be explained by its ecological existence and conducive environmental conditions allowing its optimal growth (pH, soil composition etc). Ability to form grains and provoking an inflammatory response gives it an advantage in establishing mycetomas in humans, but due to its low virulence it does not frequently contaminate wounds or cause manifestations. Of all the infections due to Acremonium species, A. kiliense is not commonly reported. As in the present case, although mycetomas are a common clinical manifestation, patients have a tendency to delay medical attention until the disease widely spreads. This patient could not distinctly recollect which of the lesions – nail or the sole, appeared earlier. The occurrence of distal onychomycosis of the toe nail preceding the involvement of the subcutaneous tissue could be a probable reason for the spread of infection from the nail plate. Moreover the patient was diabetic, poorly controlled on anti-diabetics, which may be an underlying risk factor responsible for the spread of infection from the primary site of infection (nail plate). Nevertheless, the likelihood of an unrecalled trauma, warm wet environmental conditions and habit of constant wearing of shoes, predisposing and providing a refuge for the infection cannot be ruled out. This subcutaneous hyalohyphomycosis resembled the picture of mycetoma but lacking in draining sinus tracts has been referred to as pseudomycetomas [1].
Different species of Acremonium have been attributed to varying clinical conditions. Of the 150 species known, there are nine species associated as human pathogens– A. falciforme (currently known as Fusarium falciforme), A. recifei, A. kiliense, A. potronii, A. roseogriseum, A. strictum, A. alabmensis, A. blochi, A. astrogriseum [23]. The present isolate, A. kiliense, has been differentiated from its commonest counterpart A. falciforme, on basis of shape of conidia [24]. Pale grain eumycetomas caused by Acremonium produce purulent discharge containing abundant neutrophils and white to pale-yellow soft grains granules variable in shape, which can be mimicked morphologically with similar fungi like Fusarium or Pseudalleshcheria species. However, careful microscopic examination of the hyphal and conidial elements, along with growth characteristics can minimize these differences. These fungi can be isolated on any standard mycological media. It is recommended that presence of mycotic elements in multiple specimens (from the same or related site) should be demonstrated in case of infections in immunocompetent host. Thus, owing to the complexity of various patient groups at risk for infection, opportunistic mycoses pose a considerable diagnostic challenge to both clinicians and microbiologists.
Optimal treatment of Acremonium infection is not very well documented despite approved susceptibility testing method of filamentous fungi. Very often results do not correlate with clinical cure because of inherent variability and poor reproducibility of the testing methods [17]. Despite various reports of in-vitro resistance of these fungi to azoles [6], many cases resolve with the combination of surgery (where-ever indicated) and azole derivatives given for prolonged periods. Overall data shows that amphotericin B is the most efficacious drug used for treating Acremonium infections followed by itraconazole, miconazole and ketoconazole as alternative therapeutic options [6]. Medical management therefore, has been a constant challenge for the clinicians in treating mycetomas due to Acremonium species [21]. Therefore for a favorable course and cure of Acremonium infections, choice of antifungals may vary and success of treatment may not be consistent with the same agents in all cases.
Microdilution broth method compared the sensitivity of terbinafine, fluconazole, voriconazole and amphotericin B of A. kiliense and all were found to be sensitive.Our patient was given a course of fluconazole and terbinafine for an extended duration. Although the infection did not disseminate systemically it resolved after a prolonged indolent course of eighteen months.
It brings us to observe that amongst the causative agents of hyalohyphomycosis, Acremonium species have demonstrated a significant responsibility in causing different infections, similar to fungi like Fusarium and Scedosporium species. In immunosuppressed patients these infections tend to disseminate and prove to be fatal. The current diagnostic approach including clinical suspicion, culture of appropriate samples from suitable sites, histopathological examination of relevant sections and imaging techniques becomes absolutely essential to optimize diagnosis and treatment of these infections especially in compromised hosts. Due to variable susceptibility to antifungal drugs, it is essential to identify these agents correctly and deposit them with reference laboratories for future studies.
Acknowledgements
The author’s are thankful to Dr. Arunaloke Chakraborty (Professor, Department of Medical Microbiology, Centre of Advanced Research in Medical Mycology, Post Graduate Institute of Medical Education and Research, Chandigarh, India) for confirming our isolate species and performing antifungal susceptibility of the same.
Key Points
- Hyalohyphomycosis is an unusual opportunistic mycotic infection. ‘Hyalohyphomycosis’ term is specially designated for infections caused by ubiquitous saprophytes such as Fusarium, Scedosporium, Scopulariopsis, Penicillium marneffei, Acremonium etc., which appear as hyaline, septate mycelial elements, with branched or intertwined hyphae without any pigmentation in the cell wall.
- Environmental saprophytes cause a variety of infections mostly thought to be secondary to increased host susceptibility and prior colonization.
- Subcutaneous nodules in a middle-aged diabetic patients could be attributed to hyalohyphomycosis, as observed in the present case (due to Acremonium).
|
References
- Anadolu R, Hilmioglu S, Oskay T, Boyvat A, Peksari Y, Gurgey E. Indolent Acremonium strictum infection in an immunocompetent patient. Int J Dermatol 2001; 140: 451-3.
- Lopes JO, Kolling LC, Neumaier W. Kerion like lesion of the scalp due to Acremonium kiliense in a non compromised boy. Rev Inst Med Trop Sao Paulo 1995; 37: 358-65.
- Fincher RME, Fisher JF, Lovell RD, Newman CL, Espinellngroff A, Shadomy HJ. Infection due to fungus Acremonium (Cephalosporium). Medicine (Baltimore) 1991; 70: 398-409.
- Clinical and Laboratory Standards Institute. Performance standards for antimicriobial susceptibility. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: Approved Standard. CLSI/NCCLS document M38-A [ISBN 1-56238-470-8]. 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA, 2002.
- Yalaz M, Hilmigolu S, Metin D, Akisu M, Nart D, Cetin H. Fatal disseminated Acremonium strictum infection in a preterm newborn: a very rare cause of neonatal septicemia. J Med Microbiol 2003; 52: 835-7.
- Guarro J, Gams W, Pujol I, Gene J. Acremonium species new emerging fungal opportunists’ in-vitro antifungal susceptibilities and review. Clin Infect Dis 1997; 25: 1222-9.
- Fincher SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev 1996; 9: 499-511.
- Van Etta LL, Peterson LR, Gerding DN. Acremonium falciforme (Cephalosporium falciforme) mycetoma in a renal transplant patient. Arch Dermatol 1983; 119: 707-8.
- Castro LG, Piqerro CJ. Clinical and mycological finding and therapeutic outcome of 27 mycetoma patients from Sao Paulo Brazil. Int J Dermatol 2008; 47: 160-3.
- Gever AS, Fox LP, Husain S, Della-Latta P, Grossman ME. Acremonium mycetoma in a heart transplant receipient. J Am Acad Dermatol 2006; 55: 1095-100.
- Negroni R, Lopez DG, Arechavala A, Binachi MH, Robles AM. Clinical and microbiological study of mycetomas at the Muniz hospital of Buenos Aires between 1989 - 2004. Rev Argent Microbiol 2006; 38: 13-8.
- Cordoba A, Fraenza L. Mycetoma from Acremonium species. Ann Dermatol Venereol 2005; 132: 194.
- Lacaz SC, Pereira AD, Castro MLG. Podalic mycetoma. An Bras Dermatol, Rio de Janeir 1999; 74: 591-95.
- Zaitz C, Porto E, Heins-Vaccari EM, Sadahiro A, Ruiz LR, Muller H, et al. Subcutaneous hyalohyphomycosis caused by Acremonium recifei: case report. Rev Inst Med Trop Sao Paulo 1995; 37: 267-70.
- Venugopal PV, Venugopal TV. Pale grain eumycetomas in Madras. Australas J Dermatol 1995; 36: 149-51.
- Miro O, Ferrando J, Lecha V, Campistol J. Abcessos subcutaneous por Acremonium falciforme en un transplantado renal. Med Clin (Barc) 1994; 102: 316.
- Simon G, Rakoczy G, Galgoczy J. Acremonium kiliense in esophagus stenosis. Mycoses 1991; 34: 257-60.
- Lacroix C, Jacquemin J, Guilhot F, Rabot MH, Burucoa C, de Bievre C. Septicemie a Acremonium kiliense avec dissemination secondaire chez une patiente d’un myelome a forte masse tumorale. Bull Soc Fr Mycol Med 1988; 17: 93-8.
- Koshi G, Padhye AA, Ajello L, Chandler FW. Acremonium receifei as an agent of mycetoma in India. Am J Trop Med Hyg 1979; 28: 692-96.
- Venugopal TV, Venugopal PV, Parmasivan CN, Shetty BM, Subramanium S. Mycetomas in Madras. Sabouraudia 1997; 15: 17-22.
- Halde C, Padhye A, Haley LD, Rinal diMG, Kay D, Leeper R. Acremonium falciforme as a cause of mycetoma in California. Sabouraudia 1976; 14: 319-326.
- Padhyee AA, Sukapure RS, Thirumalachar MJ. A second case of Madura foot in India caused by Cephalosporium madurae. Hindustan Antibiot Bull 1966; 8: 212-5.
- Zalas N. Superficial white onychomycosis. Sabroudia 1966; 5: 99-103.
- Gainch ML, Padhye AA, Thirumalachar MJ. Madura foot in India caused by Cephalosporium infestans species. Sabouraudia 1962; 1: 230-33.
|


