| Review Article | ||||
| Harnessing nuclear energy: health risks | ||||
| Amit Bhasin*, Aparna Ahuja**
*Department of Medicine, Lady Hardinge Medical College, New Delhi
Introduction With increasing use of nuclear energy for generating power, therapeutic medical purposes and also for nuclear terrorism, there has been also an increase in radiation accidents and hence there is need for adequate medical knowledge and response. The nuclear debate is growing in importance as governments everywhere are looking for ways to maintain economic growth and reduce the effects of global warming. But it now seems like the disadvantages are just too great. Nuclear power provides about 6% of the world's energy and 13–14% of the world's electricity, with the U.S., France and Japan together accounting for about 50% of nuclear generated electricity [1,2]. Nuclear power is controversial and there is an ongoing debate about the use of nuclear energy. Proponents, such as the World Nuclear Association and International Atomic Energy Agency (IAEA), contend that nuclear power is a sustainable energy source that reduces carbon emissions. Opponents, such as Greenpeace International and NIRS, believe that nuclear power poses many threats to people and the environment [3,4]. These threats include the problems of processing, transport and storage of radioactive nuclear waste, the risk of nuclear weapons proliferation and terrorism, as well as health risks and environmental damage from uranium mining. They also contend that reactors themselves are enormously complex machines where many things can and do go wrong, and there have been serious nuclear accidents [5,6] Determinants of radiation injury- The amount of
radiation (i.e. radiation dose) absorbed by the
patient's tissues is highly predictive of its biological
effects. Such doses are defined as the amount of energy of ionising radiation deposited per unit of
tissue mass at a specific point. Some amount of
exposure naturally occurs during certain medical
imaging such as: a standard chest x-ray delivers a
dose of 6 to 11 mrem (0.06 to 0.11 mSv, 0.06 to 0.11
mGy). Interventional cardiologists working in a highvolume
catheterisation laboratory may have collar
badge exposures exceeding 600 mrem (6 mSv) per
year. A barium enema with 10 spot images delivers a
dose of approximately 0.7 rem (700 mrem, 7 mSv, 7
mGy). Similar doses (7-8 mSv) are delivered from a
CT scan of the chest or a PET scan, while a combined
PET/CT scan is estimated to deliver a dose of 25 mSv
[7,8,9]. But these exposures are not hazardous. The
lowest radiation dose resulting in an observable
effect in man on bone marrow depression, with a Several factors determine the lethality of ionising
radiation. These include: ‘Dose rate’ (doses
received over a shorter period of time cause more
damage), ‘Distance from the source’ (For point
sources of radiation, the dose rate decreases as the
square of the distance from the source {inverse Radiation exposure causes different degree of damage to various body structures depending upon certain inherent properties of cells. Radiosensitivity varies directly with the ‘rate of cellular proliferation’. Rapidly dividing cells are more profoundly affected. Radiosensitivity varies directly with the ‘number of future divisions’. Long-lived gonadal and haematopoietic stem cells fall into this category. Radiosensitivity varies indirectly with the‘degree of morphologic and functionl differentiation’. As an example, cells at the growth plate in bone, which have not yet developed into bone or cartilage, are more sensitive than those of the diaphysis. Accordingly, growth arrest of bone is commonly seen after radiation exposure to the growth plate in children, as may occur in the treatment of malignancy. Variation in sensitivity to radiation is an inherited genetic trait, although candidate gene studies have been largely unsuccessful in identifying the genetic variants underlying most phenotypes [13,14]. While all tissues composed of short-lived cells are directly and indirectly affected by radiation, the most critically affected tissues in adults include the following: spermatocytes in the testis, haematopoietic precursor cells in the bone marrow and crypt cells in the intestines. Dose-dependent effects on various organs have also been identified. They are of two types, deterministic and stochastic: A ‘deterministic’ effect is one in which the severity is determined by the dose (e.g. depression of blood counts). A dose threshold (i.e. a dose below which an effect is not seen) is characteristic of this effect. As an example, the threshold absorbed dose for a "deterministic effect" on bone marrow (0.5 Gy) is lower than that for all other organs, except for the testis (0.15 Gy). A‘stochastic’ effect represents an outcome for which the probability of occurrence (rather than severity) is determined by the dose. An example is radiationinduced carcinogenesis, which occurs after a prolonged and variable delay (latency) after exposure. These effects do not have an apparent threshold dose. The mechanisms underlying deterministic and stochastic effects remain unknown. Studies showing the impact of radiation on gene function may shed light in this area. Radiation injury The damage caused by radiation exposure can be categorised according to whether the symptoms and signs develop immediately or are delayed by months or years. The ensuing damage results from the sensitivity of cells to radiation, with the most rapidly dividing cells being the most sensitive to the acute effects of radiation. The inherent sensitivity of these cells results in a constellation of clinical syndromes that occur within a predictable range of doses after a whole-body or significant partial-body exposure. Symptoms arising from such exposures are referred to as radiation sickness or acute radiation syndrome (ARS). Classically, the threshold dose for ARS is a whole-body or significant partialbody irradiation of greater than 1 Gy delivered at a relatively high dose rate. Acute radiation syndrome Acute changes, which are seen within the first two months following exposure, include signs and symptoms resulting mainly from damage to the skin, central nervous system, lung, gastrointestinal tract, and haematopoietic tissues. Classic clinical syndromes associated with ARS include the haematopoietic, gastrointestinal, and cerebrovascular (formerly known as cardiovascular and central nervous) syndromes, although there is significant clinical overlap. Local radiation injury, sometimes called the
Cutaneous Syndrome (CS), is especially common and
important in patients with ARS consequent to a nonuniform
exposure. The CS may include changes
ranging from epilation to radionecrosis. The
presence of ARS complicates the management of CS, There are four main phases to the ARS [16]-
The Prodromal Phase usually occurs in the first 48
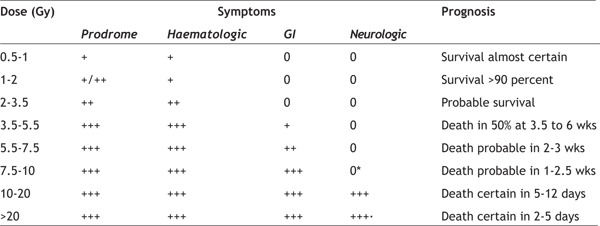
hours following exposure, but may develop up to six The Latent Phase is a short period characterised by improvement of symptoms. However, this effect is transient, lasting for several days to a month. The duration of this phase is inversely related to the dose of radiation received, and may be absent at the highest, fatal doses. The Stage of Manifest Illness may last for weeks, and is characterized by intense immunosuppression. It is the most difficult to manage. If the person survives this stage, recovery is likely. Death or Recovery Phase — Those patients who recover will require close follow-up for the first year, owing to the risk for unusual infections, as aberrant immune reconstitution is probable in those with significant exposure. Survivors will require lifelong follow-up to monitor for long-term complications, such as organ dysfunction and carcinogenesis. The onset, duration, and dominant pattern of the acute radiation syndrome depend upon the dosage of radiation received (Table 1) [17,18]. As examples, the prodromal syndrome is often minimal in those exposed to doses of ≤ 1 Gy, while those exposed to doses of 10 to 20 Gy may have a rapid compression of phases and proceed from the prodromal phase to death in two days or less. Table 1- Phases of radiation injury
The Prodromal Syndrome is generally mild or absent
at total body doses of 1 Gy or less. Patients whose
symptoms begin more than two hours after exposure
were probably exposed to doses <2 Gy. They can be
expected to fully recover within one month,
although long-term sequelae may develop. Onset of
symptoms within the first two hours usually
indicates significant and potentially lethal
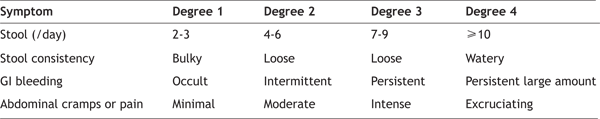
exposures exceeding 2 Gy. At these doses, sloughing The Cerebrovascular Syndrome, also called the neurovascular syndrome or CNS syndrome, results from localised changes in the central nervous system. These include impaired capillary circulation with damage to the blood-brain barrier, interstitial oedema, acute inflammation, petechial haemorrhages, inflammation of the meninges, and hypertrophy of perivascular astrocytes. Paroxysmal spike and wave discharges may be evident on the EEG, and the presence of swelling and oedema may be documented by CT scan and MRI of head [22]. There may be a latent period of a few hours in which there is apparent improvement, but within five to six hours watery diarrhoea, secondary to severe gastrointestinal syndrome, respiratory distress, fever, and cardiovascular collapse ensue. The final picture, which may mimic that of sepsis, includes hypotension, cerebral oedema, increased intracranial pressure, and cerebral anoxia, with death in about two days time. The Gastrointestinal Syndrome typically develops within five days of the initial exposure (Table 2) [18]. At doses <1.5 Gy, only the prodromal phase of nausea, vomiting, and gastric atony are observed [23]. More severe symptoms develop at doses between 5 and 12 Gy, secondary to loss of intestinal crypt cells and breakdown of the mucosal barrier, with sloughing of the epithelial cell layer and denudation of the bowel wall [24]. These changes result in crampy abdominal pain, diarrhoea, nausea and vomiting, gastrointestinal bleeding with resultant anaemia, and abnormalities of fluid and electrolyte balance. This early phase is often followed by a latent phase lasting five to seven days, during which symptoms abate. Vomiting and severe diarrhoea accompanied by high fever make up the manifest illness. Systemic effects at this time may include malnutrition from malabsorption. Table 2- Radiation toxicity- gastrointestinal system
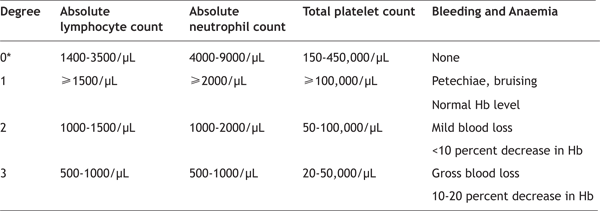
Impaired barrier function of the gastrointestinal tract results in the passage of bacteria and their toxins through the intestinal wall into the bloodstream, predisposing to infection and sepsis, which may be further compromised by immunosuppression and cytopenias (secondary to development of the haematopoietic syndrome). Other severe complications include ulceration and necrosis of the bowel wall, leading to stenosis, ileus, and perforation. In the latter case, recovery is most unlikely, as radiosensitive stem cells in the crypts of the gastrointestinal tract are permanently damaged. Consequently, there is no replacement of cells that are lost from the surface of the villi through the sloughing process, precluding recovery [25]. However, mild gastrointestinal symptoms limited to one or two episodes of diarrhoea with associated abdominal pain are accompanied by virtually certain recovery, provided that the haematopoietic syndrome which follows is reversible. The Haematopoietic Syndrome develops at doses exceeding 1 Gy and is rarely clinically significant at doses <1 Gy [20,21,24,26,27]. Mitotically active haematopoietic precursors have limited capacity to divide after whole-body doses greater than 2 - 3 Gy. Neutropenia and thrombocytopenia reach a nadir at two to four weeks and may persist for months. Anaemia inevitably ensues, due to the combined effects of gastrointestinal blood loss from the gastrointestinal syndrome, haemorrhage into organs and tissues secondary to thrombocytopenia, and ultimately, bone marrow aplasia. In the ensuing weeks to months after exposure, hypoplasia or aplasia of the bone marrow occurs, resulting in pancytopenia, predisposition to infection, bleeding, and poor wound healing, all of which may contribute to death in the absence of appropriate supportive care. Lymphopenia is common and occurs before depression of other cellular elements, and may develop within the first 6 to 24 hours after exposure to a moderate or high dose [25,28,7]. Based on the overall levels of lymphocyte, neutrophil, and platelet counts, as well as the presence or absence of infection and blood loss, the relative severity of toxicity to the haematopoietic system can be evaluated (Table 3) [18]. Table 3- Levels of haematopoietic toxicity following radiation exposure
The Cutaneous Syndrome may develop early following exposure (e.g. one to two days). However, it may take years before becoming fully manifest. Early lesions include erythema, oedema, and dry desquamation of the skin. Such lesions may be isolated or may appear simultaneously in several locations, depending on the amount of skin receiving direct exposure. More advanced lesions include bullae, moist desquamation, ulceration, and onycholysis. The severity of the cutaneous reaction depends upon the depth dose distribution of the radiation source.
Delayed effects Long Term Radiation Exposure results from residing in a fallout contaminated area for an extended period (external exposure), consuming food produced in a contaminated area (internal exposure), or both. If the exposure rate is low enough, no symptoms of radiation sickness will appear even though a very large total radiation dose may be absorbed over time. Latent radiation effects (i.e. cancer, genetic damage) depend on total dosage, not dose rate, so serious effects can result. Internal Exposure: Radioisotopes may be taken up into plants through the root system, or they may be contaminated by fallout descending on the leaves. The primary risks for internal exposure are caesium-137 and strontium-90. Strontium-89, transuranics alpha emitters, and carbon-14 are also significant sources of concern. Caesium-137 is readily absorbed by food plants, and by animal tissues and distributes itself fairly evenly through the body causing whole body exposure. Strontium is chemically similar to calcium, and is deposited in bone along with calcium. Somewhat less than 10% of the strontium is retained in the bone, but since bone marrow is among the most sensitive tissue in the body to radiation, this creates a very serious hazard. If small particles of alpha emitters are inhaled, they can take up permanent residence in the lung and form a serious source of radiation exposure to the lung tissue. Uranium and the transuranic elements are bone-seekers (with the exception of neptunium) and present a serious exposure risk to bone tissue and marrow. Cancer: The most serious long term consequence of radiation exposure is the elevation of cancer risk. Cancer risk is more or less proportional to total radiation exposure, regardless of the quantity, rate or duration. There is no evidence of a "safe dose". Safety standards are established primarily to keep the increased incidence of cancer below detectable levels. Cancer risk to radiation exposure can be expressed as the increase in the lifetime probability of contracting fatal cancer per unit of radiation. The current estimate of overall risk is about a 0.8% chance of cancer per 10 rem for both men and women, averaged over the age distribution of the U.S. population. There is also risk coefficient for specific tissue exposures (approximately):
Genetic Effects: Radiation damage to germ cells of the reproductive organs can cause mutations that are passed on to subsequent generations. However, no elevated mutation rate from radiation has ever been detected even in the substantial population of atomic bomb survivors and descendants. Two factors can explain this- High acute exposures to the reproductive organs can cause permanent sterility, which prevents transmission of genetic effects and cumulative effect of chronic exposure is limited by the fact that only exposures prior to reproduction count. Since most reproduction occurs before the age of 30, exposures after that age have little effect on the population. Cataracts: Eye tissues exposed to radiation show an increased incidence of cataracts at dose levels below which most tissues show increased cancer rates. This makes cataract risk the most important tissue dose criterion for establishing safety standards [31]. Source of information Our understanding of the effects of total-body radiation is derived from analysis of the clinical course of individuals exposed to radiation after the detonation of two atomic bombs over Japan in 1945, as well as radiation accidents that have occurred throughout the world since that time. In some cases, this includes a large affected population (e.g. the Marshallese exposed in 1954 and individuals in the former Soviet Union and Europe exposed during the Chernobyl nuclear power plant disaster in 1986). As examples: the Chernobyl reactor explosion in the former Soviet Union resulted in high levels of exposure, with 28 people receiving doses >6 Gy, 23 receiving 4 to 6 Gy, and 53 receiving 2 to 4 Gy [32]. There were 115 cases of acute radiation syndrome and 28 deaths. In 1987, in Goiania, Brazil, an abandoned Caesium-137 teletherapy source was breached, with hundreds of people exposed to gamma and beta radiation [33]. There were 48 hospitalisations for radiation injury and four deaths. In contrast, the reactor breach at Three Mile Island in the United States was calculated to result in no more than 50 to 70 mrem of additional exposure to any individual within range. In other cases, relatively low numbers of individuals have been exposed. The Chernobyl nuclear power plant led to
radioactive contamination of aquatic systems which
became a major problem in the immediate
aftermath of the accident [34]. In the most affected
areas of Ukraine, levels of radioactivity
(particularly radioiodine: I-131, radiocaesium: Cs-
137 and radiostrontium: Sr-90) in drinking water caused concern during the weeks and months after
the accident. Bio-accumulation of radioactivity in
fish was seen to be significantly above guideline
maximum levels for consumption [34]. Groundwater was not badly affected by the Chernobyl accident
since radionuclides with short half-lives decayed
away long before they could affect groundwater
supplies, and longer-lived radionuclides such as
radiocaesium and radiostrontium were adsorbed to
surface soils before they could transfer to
groundwater [35]. However, significant transfers of
radionuclides to groundwater have occurred from
waste disposal sites in the 30 km exclusion zone
around Chernobyl. After the disaster, four square In April 2010, a 35 year old man died after exposure to scrap metal containing Cobalt-60 in the Mayapuri industrial area of New Delhi [37]. The man died of multiple organ failure when various treatment modalities failed to resuscitate him. Six other people from the same area were also hospitalised after being exposed to the contaminated scrap metal. Officials retrieved 11 samples of contaminated materials containing Cobalt-60, which is a radioactive material used in food irradiation and radiotherapy. Following an earthquake, tsunami, and failure of
cooling systems at Fukushima I Nuclear Power Plant
and issues concerning other nuclear facilities in
References
|
||||