| Shruti Tandon*, Mandeep Singh Dhingra*, Arundeep Kaur Lamba*, Mahesh Verma**, Akshay Munjal*, Farrukh Faraz*
*Department of Periodontics, **Department of Prosthodontics, Maulana Azad Institute of Dental Sciences, MAMC complex,
New Delhi-110002, India.
Corresponding Author: Dr. Shruti Tandon, Asst. Professor, Department of Periodontics, 6th floor Maulana Azad Institute of
Dental Sciences, MAMC complex, New Delhi-110002, India. Fax No. 011-23217081. Email: drst@in.com
Abstract
Background & objectives: Periodontitis is a destructive inflammatory disease of the supporting tissues of the
teeth that occurs in response to a predominantly Gram-negative bacterial infection originating from dental
plaque. Periodontitis presents with increased systemic inflammation and is known to contribute to rise in
serum lipid levels. The aim of present study was to determine influence of periodontal therapy on serum lipid
levels.
Methods: A total of 105 consecutive subjects were studied. Group I (n=35) included subjects with chronic
generalised periodontitis who were not given periodontal therapy during study period and served as control
group. Group II (n=70) comprised of subjects with chronic generalized periodontitis who were rendered
needful periodontal therapy. Serum levels of triglycerides, total cholesterol, HDL & LDL cholesterol were
measured at day 0 (baseline) and reassessed on day 60.
Results: In the treatment group, serum triglycerides (Pre=127.81±59.32 & Post = 121.20 ± 58.94 mg/dL, p <
0.001)), total cholesterol (Pre=176.33±38.31 & Post=171.39±31.19 mg/dL, p=0.045) and mean LDLcholesterol
levels (Pre=91.91±28.54 & Post=83.94±26.00 mg/dL, p < 0.001) showed a significant decline from
the pre-treatment values. HDL-cholesterol levels did not change significantly in both groups. Other lipid
levels were not significantly altered in the control group.
Conclusions: Patients of chronic generalised periodontitis who were offered periodontal therapy showed
improvement in the various lipid parameters except HDL-cholesterol, which was not significantly altered.
Chronic periodontitis in otherwise healthy individuals may therefore, be contributing to the systemic
inflammatory burden in these patients and adversely altering the lipid profile.
Key words: Cardiovascular disease, Inflammation, Periodontitis, Risk factor
|
Introduction
Periodontitis represents a chronic oral infection that
leads to gingival inflammation, destruction of toothsupporting
tissues, namely periodontal ligament and
alveolar bone and eventual exfoliation of teeth. The
etiological agents for periodontitis are the Gramnegative,
anaerobic micro-organisms present within
the dental plaque. These micro-organisms produce
endotoxins in the form of lipopolysaccharides (LPS)
that are instrumental in generating a host-mediated
immune response. LPS gain access to the gingival
tissue and stimulate an inflammatory response
characterised by infiltration of neutrophils,
lymphocytes, macrophages and mast cells [1]. The
net effect of this stimulation is the production of Interleukin-I α and β (IL-I α and β), Tumour Necrosis
Factor α (TNF- α), IL-6 (all of which can stimulate
bone resorption) and matrix metalloproteinases (a
group of calcium & zinc dependent enzymes) which
digest collagen. Therefore it is this host-mediated
immune response that eventually leads to
periodontal tissue destruction.
Recently there has been great interest in the
systemic effects of pro-inflammatory cytokine
levels in serum, potentially elevated by
periodontitis. Evidence suggests that low level,
chronic exposure to gram negative microorganisms
and/or their LPS can manifest a state of altered lipid
metabolism; the main features of which are
hypertriglyceridemia and lipid oxidation. The underlying mechanism for these alterations is the
release of TNF-α and Il-1 β in response to Gramnegative
LPS exposure. These two cytokines exert
effects on lipid metabolism by influencing the
production of other cytokines [2,3], altering
hemodynamics/ amino acid utilization of various
tissues involved in lipid metabolism [4,5] or
modifying the hypothalamic–pituitary–adrenal axis,
increasing plasma concentrations of
adrenocorticotropic hormone (ACTH), cortisol,
adrenaline, nor-adrenaline and glucagon [6,7]. The
above modifications in turn, lead to enhanced
hepatic lipogenesis [8], increased synthesis or
reduced clearance of triglycerides [9,10] and
reduced clearance of LDL due to reduced
lipoprotein lipase activity [11,12].
Studies have suggested that in advanced
periodontitis, levels of IL- 1β and TNF-α are
sufficiently elevated in gingival crevicular fluid
(GCF) to be “dumped” systemically, falling within
the detectable range of biological serum assays [13,14,15]. This increase in plasma concentrations of
cytokines leads to a state of altered lipid
metabolism. This observation in the plasma levels of
IL- 1β and TNF-α has the potential to trigger the
mechanisms that lead to hyperlipidemia.
Consequently, resolution of inflammation caused by
periodontitis by imparting therapy; is expected to
reduce the serum lipid levels. The present study was
carried out in the above background, with the aim of
evaluating the effect of periodontal therapy on
serum lipid levels.
Materials and Methods
Patient selection & study design: Consecutive 105
subjects in the age range of 35-55 years (mean age
42.89±5.66 yrs), sufferring from periodontitis were
selected from outdoor patient department of
Periodontics at Maulana Azad Institute of Dental
Sciences, New Delhi, irrespective of sex, religion
and socioeconomic status. The other inclusion
criteria was the presence of a minimum of 24 teeth
and a probing depth of 5 mm or more with a clinical
attachment loss (CAL) of ≥ 3mm in at least 30% sites.
There were 67 (63.8%) males and 38 (36.2%) females
in study. Subjects with known systemic ailments, or
having any other oral lesion, or with a positive
history of any periodontal therapy within past 6
months or history of antibiotic use within past three
months were excluded from the study. Pregnant
women or those planning pregnancy and smokers too were excluded from the study. The design,
duration and purpose of the study was discussed
with the subjects and written informed consent was
obtained from them. The smaller sample size in the
control group is because fewer patients consented
to be off-therapy as compared to a larger number
who consented to have therapy. Ethical approval
was obtained from the institutional review board.
At baseline, for each subject, the periodontal
disease status was evaluated at 4 sites per tooth
(mesiobuccal, buccal, distobuccal and lingual) by a
single trained periodontist using UNC-15 probe (Hu-Friedy's, USA). The Gingival Index - GI (Loe and
Silness, 1963), Probing Pocket Depth (PD) and
Clinical Attachment Level (CAL) was recorded.
Thereafter, for the baseline assessment of serum
lipids, blood samples were taken.
All subjects were called early morning after 12 hours
of fasting. 4 ml of blood was drawn by venous
puncture from each of the subjects following the
standard protocol for the same. All the samples
were collected in the serum separation vacutainers.
Samples were centrifuged in the centrifuge machine
at 2500-3000 rpm for 8- 10 minutes to separate the
serum from the blood. Separated serum was
collected in appendox and stored in deep freeze at -
50 degree centigrade. These specimens were
stored so that the evaluation of samples which
would be taken on the completion of the study could
be done simultaneously to minimize inter-kit error.
The subjects were divided in to two groups-
Group I: Subjects in this group (n=35) did not
receive any treatment during the study period and
served as control population. Their blood samples
were taken and periodontal status was recorded at
baseline. They were not given any kind of
medication; neither were they taught about the
brushing method nor about other oral hygiene
measures. These subjects were recalled 2 months
after their initial blood samples were taken, for
obtaining second blood sample in accordance with
protocol followed at the baseline.
Group II: The patients in this group (n=70) were
imparted therapy for periodontal disease. The
periodontal therapy rendered comprised of full
mouth disinfection that included mechanical plaque
control, together with full mouth scaling and root
planning under local anaesthesia. The patients were
placed on strict oral hygiene maintenance programme. The effect of phase I therapy was
evaluated after 1 month and in case the residual
periodontal pockets were present, surgical
procedure was performed in the required sites. The
number of sites receiving surgical procedures varied
from patient to patient. Regular recall was made
after 2-3 weeks to check oral hygiene maintenance.
Their second blood sample was collected after two
months of initiating active periodontal therapy.
Statistical analysis:
The data collected was subjected to statistical analysis to evaluate the
effect of periodontal intervention on the lipid
profiles of the subjects in the two groups. Data
obtained was analysed by SPSS version 13.0
statistical software and p-value less than 5% was
taken as significant. Association between gender
and study groups was tested by Chi-square test.
Unpaired Student’s t-test was used to compare all
the baseline parameters (age, sex, triglycerides,
total, HDL & LDL-cholesterol) between the two
groups i.e. control and treatment group. The change
in the values of lipid profile parameters (pre-post)
were also compared by the unpaired Student’s ttest.
Paired students t-test was performed to
compare the pre and post values of triglycerides,
total, HDL & LDL-cholesterol separately for each
group.
Results
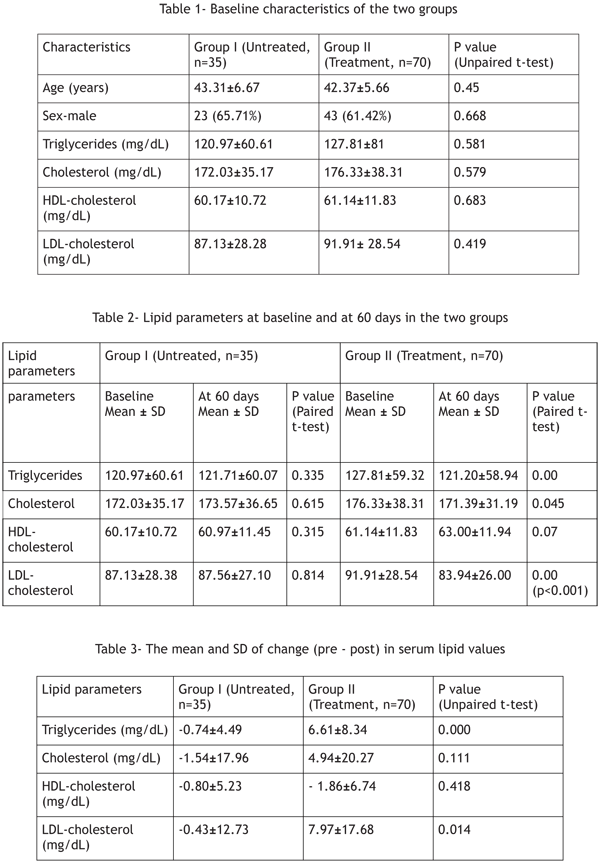
Table 1 outlines the summary of baseline
parameters in the two groups, which were similar.
Table 2 details the comparison between pre and post
treatment measurements of serum lipid parameters
in the two groups. In the treatment group, average
serum triglycerides, total cholesterol and LDLcholesterol
values showed significant reduction
from the pre-treatment levels. Mean HDL was
increased by 1.86 mg/dL from pre-treatment value
but this increase was not significant at 0.05 level of
significance (p=0.070). However in the control
group average values of various lipid parameters did
not show any significant change.
Table 3 shows the mean and standard deviation (SD)
of change (pre - post) of values in triglycerides, total
cholesterol, HDL and LDL-cholesterol values. A
statistically significant decrease in the mean
triglycerides and LDL-cholesterol values was
observed in the treatment group vis-à-vis the
control group. The change in total cholesterol did not reach statistical significance between the two
groups. Although the HDL values increased in both
the groups, but there was no statistically significant
difference between the changes in the two groups.
Discussion
Over the last 50 years, the prevailing view among
dentists and physicians was that periodontal
infections were localized only to the marginal
periodontium and rarely had systemic implications
in healthy individuals. More recent evidence,
however, has shown that patients with periodontitis
present with increased systemic inflammation, as
indicated by raised serum levels of various
inflammatory markers when compared with those in
unaffected control populations [16,17,18,19]. It is also
well known that there is a causal relationship
between serum lipid levels and systemic health,
particularly cardiovascular disease, diabetes, tissue
repair capacity, immune cell function, and serum
levels of proinflammatory cytokines. A significant
association between periodontitis and cholesterol
has been reported [20].
The present study evaluated the effect of
periodontal disease on the serum lipid levels and
found that periodontal therapy resulted in
significant decrease in the levels of serum total
cholesterol, trigycerides and LDL-cholesterol. The
subjects included in this study had no major
differences in possible confounders when the two
groups were compared.
Periodontal disease is now recognised to produce
numerous changes in systemic health, changing the
blood chemistry with a rise in inflammatory
mediators, proteins and lipids in the serum. These
factors explain, at least in part, the probable
association between periodontitis and the
susceptibility for certain systemic diseases, such as
the increased risk of cardiovascular disease that is
highly prevalent in the world.
Acute systemic or local chronic infections seem to
induce changes in the plasma concentration of
cytokines and hormones, which determine changes
in the lipid metabolism. The study by Feingold et al
[8] showed that the administration of low doses of
endotoxins in rats resulted in hypertriglyceridemia,
suggesting the presence of a similar response in
local infections such as periodontal disease, in
which there is a chronic systemic exposure to microorganisms and lipopolysaccharides. The study
of Memon et al [21] proved that the induction of
periodontitis by Porphyromonas gingivalis in rats
resulted in an increased level of triglycerides. And
more recently, using similar methodology, the same
result was observed in another work [22].
The results of the present study are in concurrence
with the study by Katz et al [20] on the association
between hypercholesterolemia, cardiovascular
disease and severe periodontal disease and
Moeintaghavi et al [23] on hyperlipidemia in
patients with periodontitis. According to the results
of these studies, patients with periodontitis had
significantly higher levels of triglycerides and
cholesterol.
The studies which evaluated the effect of
periodontal therapy on serum lipids and lipoprotein
associated inflammatory mediators also suggested
that the treatment of periodontal disease has
beneficial effects on lipid metabolism. In one study
conducted in systemically healthy subjects with
periodontitis, Pussinen et al [24] stated that
periodontitis is associated with macrophage
activation via increased serum LPS concentration.
Additionally, there was a significant increase in the
ratio of HDL/LDL after periodontal treatment in this
study. In another study, Pussinen et al [25] reported
that there were statistically significant decreases in
CRP and serum amyloid A levels after periodontal
treatment in systemically healthy subjects with
periodontitis. That study also suggested that
periodontitis diminishes the anti-atherogenic
potency of HDL and increases the risk for coronary
heart disease.
Lösche et al [26] and Cutler et al [27] analysed the
total and LDL-cholesterol levels and triglycerides of
individuals with periodontal disease and reported
that their plasma levels were significantly higher
than healthy individuals.
Lösche et al [26] evaluated 32 patients with
moderate to severe periodontitis before and 3
months after local periodontal treatment and
reported significant reduction in the serum activity
of lipoprotein-associated phospholipase A (an 2
independent cardiovascular risk factor) with
treatment. In a similar study [28], 65 subjects
presenting with severe generalized periodontitis
were assessed. Subjects were divided into 3 groups,
consisting of untreated control; standard periodontal therapy; and an intensive periodontal
treatment including standard periodontal
treatment with adjunctive local delivery of
minocycline. In that study both standard
periodontal therapy and intensive periodontal
therapy resulted in significant reductions in serum
C-Reactive Protein (CRP) compared with the
untreated contro;l and the intensive periodontal
therapy group also showed a decrease in total and
LDL cholesterol after 2 months following the
periodontal treatment.
Higher serum levels of total cholesterol, LDL and
triglycerides have been found in subjects with
periodontal disease, and hyperlipidemic patients
have a significantly higher percentage of sites with
probing depth greater than 3.5 mm than subjects
with normal metabolic status [29]. The
interrelationship between periodontitis and
hyperlipidemia provides an example for systemic
disease predisposing to oral infection, and once the
oral infection is established, it exacerbates
systemic disease.
The underlying mechanism may be the
inflammatory local production of cytokines (IL-1,
TNF-α) and its effect on other systemic mediators
(IL-6) might induce alterations of lipid metabolism,
such as increased LDL and triglycerides, due to
increased hepatic lipogenesis, lipolysis from
adipose tissue, or reduced blood clearance [30].
Bacterial toxins (LPS) can also induce changes in
cholesterol concentrations [31]; leading to
reduction in HDL and increase in LDL-cholesterol
[32].
The finding that periodontal therapy brought about
significant changes in the lipid profiles of study
subjects reinforces the hypothesis that there exists
a relationship between periodontitis and
cardiovascular disease. This indicates that severe,
generalized periodontitis in the otherwise healthy
individuals contributes to the systemic
inflammatory burden predisposing them to
cardiovascular disease. Proposed mechanistic
explanations include: (i) the local, infection-driven
production of inflammatory mediators (IL-1, IL-6)
'dumped' into the systemic circulation [32] (ii) the
ability of periodontal pathogens and/or their toxins
to disseminate and thus induce a distant
inflammatory response and (iii) a combination of
the above.
Limitations have been the undesired companion of every study and by that very virtue they open the
gateways for further research. The present study
inevitably had a great number of subject variables
involved such as physical activity, food habits, socioeconomic
conditions, obesity, age, stress and
lifestyle which differ in accordance with the
environment in which the individual lives. These
variables are difficult to control and may have
influenced the results.
The present study reveals that subjects suffering
from periodontitis, who were rendered periodontal
therapy showed improvement in their serum lipid
parameters; which in effect goes one step ahead of
the proven correlation that exists between
periodontitis and serum lipid levels.
Key Points
- A relationship exists between periodontal
disease and serum lipid levels within the
population at large.
- Subjects with chronic generalised
periodontitis who were rendered periodontal
therapy showed significant decrease in mean
cholesterol, triglycerides and LDL values.
- This has a substantial clinical relevance in
helping to explain circumstances in which an
intra oral source of infection can create a
systemic inflammatory response, therefore
placing “apparently healthy” patients at
increased risk of cardiovascular disease.
|
Acknowledgments
Authors express their gratitude to the staff of
department of biochemistry Maulana Azad Medical
College for their help in the evaluation of serum
lipid levels. The authors thank the Indian Council of
Medical Research, New Delhi for the financial
assistance provided for this study.
References
- De Nardin E. The role of inflammatory and
immunological mediators in periodontitis and
cardiovascular disease. Ann Periodontol
2001;6:30-40.
- Socransky SS, Haffajee AD. The bacterial
etiology of destructive periodontal disease:
current concepts. J Periodontol 1992;63: 322-31.
- Page RC. The role of inflammatory mediators in
the pathogenesis of periodontal disease. J
Periodontal Res 1991;26:230-42.
- Moldawer LL. Biology of proinflammatory
cytokines and their antagonists. Crit Care Med
1994;22:S3-7.
- Fukushima R, Saito H, Taniwake K. Different
roles of IL-1 and TNF on hemodynamics and
interorgan amino acid metabolism in awake
dogs. Am J Physiol 1992;262:E275-81.
- Imura H, Fukata J, Mori T. Cytokines and
endocrine function: an interaction between the
immune and neuroendocrine systems. Clin
Endocrinol 1991;35:107-15.
- Chrousos GP. The hypothalamic-pituitarya
drenalaxis and immune-mediated
inflammation. N Engl J Med 1995;332:1351-62.
- Feingold KR, Grunfeld C. Tumor necrosis factoralpha
stimulates hepatic lipogensesis in the rat
in vivo. J Clin Invest 1987;80:184-90.
- Divertie GD, Jensen MD, Miles JM. Stimulation of
lipolysis in humans by physiological
hypercortisolemia. Diabetes 1991;40:1228-32.
- Kurpad A, Khan K, Calder AG, Coppack S, Frayn
K, Macdonald I, et al. Effect of noradrenaline on
glycerol turnover and lipolysis in the whole body
and subcutaneous adipose tissue in humans in
vivo. Clin Sci 1994;86:177-84.
- Fried SK, Zechner R. Cachectin/tumor necrosis
factor decreases human adipose tissue
lipoprotein lipase mRNA levels, synthesis, and
activity. J Lipid Res 1989;30:1917-23.
- Lanza-Jacoby S, Tabares A, Triglyceride kinetics,
tissue lipoprotein lipase, and liver lipogensesis
in septic rats. Am J Physiol 1990;258:678-85.
- Offenbacher S, Jared HL, O’reilly PG, Wells SR,
Salvi GE, Lawrence HP, et al. Potential
pathogenic mechanisms of periodontitis
associated pregnancy complications. Ann
Periodontol 1998;3:233-50.
- Prabhu A, Michalowicz BS, Mathur A. Detection
of local and systemic cytokines in adult
periodontitis. J Periodontol 1996;67:515-22.
- Salvi GE, Brown CE, Fujihashi K, Kiyono H, Smith
FW, Beck JD, et al. Inflammatory mediators of
the terminal dentition in adult and early onset
periodontitis. J Periodontal Res 1998;33:212-25.
- Kweider M, Lowe GD, Murray GD, Kinane DF,
McGowan DA. Dental disease, fibrinogen and
white cell count; links with myocardial
infarction? Scott Med J 1993:38:73-4.
- Ebersole JL, Machen RL, Steffen MJ, Willmann
DE. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis.
Clin Exp Immunol 1997;107:347-52.
- Loos BG, Craandijk J, Hoek FJ, Wertheim-van
Dillen PM, van der Velden U. Elevation of
systemic markers related to cardiovascular
diseases in the peripheral blood of periodontitis
patients. J Periodontol 2000; 71:1528-34.
- Noack B, Genco RJ, Trevisan M, Grossi S, Zambon
JJ, De Nardin E. Periodontal infections
contribute to elevated systemic C-reactive
protein level. J Periodontol 2001;72:1221-7.
- Katz J, Flugelman MY, Goldberg A, Heft M.
Association between periodontal pockets and
elevated cholesterol and low density lipoprotein
cholesterol levels. J Periodontol 2002; 73:494
500.
- Memon RA, Grunfeld C, Moser AH, Feingold KR.
Tumor necrosis factor mediates the effects of
endotoxin on cholesterol and triglyceride
metabolism in mice. Endocrinology
1993;132:2246-53.
- Doxey DL, Cutler CW, Iacopino AM. Diabetes
prevents periodontitis–induced increases in
gingival platelet derived growth factor-B and
interleukin-1 beta in a rat model. J Periodontol
1998;69:113-9.
- Moeintagahvi A, Haerian-Ardakani A, Talebi-
Ardakani M, Tabatabaie I. Hyperlipidemia in
patients with periodontitis. J Contemp Dent
Pract 2005;6:78-85.
- Pussinen PJ, Vilkuna-Rautiainen T, Alfthan G,
Palosuo T, Jauhiainen M, Sundvall J, et al. Severe
periodontitis enhances macrophage activation
via increased serum lipopolysaccharide. Arterioscler Thromb Vasc Biol 2004;24:2174-80.
- Pussinen PJ, Jauhiainen M, Vilkuna-Rautiainen
T, Sundvall J, Vesanen M, Mattila K, et al.
Periodontitis decreases the antiatherogenic
potency of high density lipoprotein. J Lipid Res
2004;45:139-47.
- Losche W, Karapetow F, Pohl A, Pohl C, Kocher T.
Plasma lipid and blood glucose levels in patients
with destructive periodontal disease. J Clin
Periodontol 2000;27:537-41.
- Iacopino AM , Cutler CW. Pathophysiological
relationships between periodontitis and
systemic disease: Recent concepts involving
serum lipids. J Periodontol 2000:71:1375- 84.
- D’Auito F, NIbali L, Parkar M, Suvan J, Tonetti
MS. Short-term effects of intensive periodontal
therapy on serum inflammatory markers and
cholesterol. J Dent Res 2005;84:269-73.
- Noack B, Jachmann I, Roscher S, Sieber L,
Kopprasch S, Luck C, et al. Metabolic diseases
and their possible link to risk indicators of
periodontitis. J Periodontol 2000;71:898–903.
- Uchiumi D, Kobayashi M, Tachikawa T, Hasegawa
K. Subcutaneous and continuous administration
of lipopolysaccharide increases serum levels of
triglyceride and monocyte chemoattractant
protein-1 in rats. J Periodontal Res 2004;39:120-8
- Offenbacher S, Farr DH, Goodson JM.
Measurement of prostaglandin E in crevicular
fluid. J Clin Periodontol 1981;8:359-67.
- Graves DT. The potential role of chemokines and
inflammatory cytokines in periodontal disease
progression. Clin Infect Dis 1999;28:482-90.
|

