Introduction
Hepatitis B virus (HBV) infection constitutes one of the major global public health problems. About 30 percent of the world's population has serological evidence of current or past infection with HBV. Chronic HBV infection is a serious clinical problem in the Asian- Pacific region where the prevalence of HBV is high. In this part of the world, the majority of HBV infection prevalence is acquired perinatally or in early childhood, and some patients may be superinfected with other viruses that may influence the clinical outcomes.
Epidemiology
Hepatitis B surface antigen positivity (HBsAg) prevalence among general population ranges from 0.1% to 11.7%, being 2% to 8% in most studies. HBsAg prevalence rate among blood donors ranged from 1% to 4.7% with the exception of higher HBsAg positivity in some North Eastern states (~7%) [1].
A large study involving 8575 pregnant women from Northern India, documented HBsAg carrier rate in antenatal mothers to be 3.7%, HBeAg carrier rate 7.8% and vertical transmission was observed in 18.6% [2]. A serological survey on 722 family members of 215 HBV infected index cases of eastern India revealed that intrafamilial horizontal transmission is the more significant mode of transmission than sexual mode of transmission in later life for maintaining HBV carrier pool in this community [3].
Hepatitis B virus has been classified into at least eight genotypes on the basis of an intergroup divergence of 8% or more in the complete genome nucleotide sequence. Subtypes are identified within some genotypes, but their clinical significance remains to be determined. Each genotype has its distinct geographical and ethnic distribution, worldwide and within the Asian-Pacific region. HBV genotypes B and C are prevalent in East and South-East Asia, the Pacific Islands, and Pakistan, whereas HBV genotypes D and A are prevalent in India. In general, genotype B is associated with less progressive liver disease than genotype C, and genotype D has a less favorable prognosis than genotype A.
Pathophysiology
The natural course of chronic HBV infection in this geographic region can be divided into
- immune-tolerant phase,
- immune clearance phase, and
- residual or inactive phase. HBV reactivation and relapse of hepatitis may occur in some patients who are in the residual or inactive phase.
Patients in the immune-tolerant phase are usually young, hepatitis B envelope antigen (HBeAg) seropositive with high viral loads 107–108 copies/mL) but normal serum alanine aminotransferase (ALT) levels and no or minimal clinicopathological changes. During the immune clearance phase, hepatitis activity and even acute flares with serum ALT levels over 5 times upper limit of normal (ULN) may occur, and these may sometimes be complicated by hepatic decompensation. Higher ALT levels, therefore, usually reflect more vigorous immune response against HBV and more extensive hepatocyte damage [4]. This is eventually followed by HBeAg seroconversion to its antibody (anti-HBe) and/or undetectable HBV-DNA. The estimated annual incidence of spontaneous HBeAg seroconversion was 2–15%, depending on factors such as age, ALT levels, and HBV genotype [4,5]. HBeAg seroconversion is followed by clinical remission (inactive chronic HBV infection) in the majority of patients. However, active hepatitis may relapse due to HBeAg seroconversion or occurrence of HBeAg-negative hepatitis. The estimated annual incidence of hepatitis relapse was 2.2–3.3% [6,7], being higher in males, genotypes C infected, and those who have HBeAg seroconversion after age 40 [8]. Spontaneous HBsAg seroclearance may occur after HBeAg seroconversion. A recent 11-year follow-up study in 1,965 asymptomatic anti-HBe positive subjects [age 16–76 years, median 34years) showed an annual HBsAg seroclearance rate of 1.2%. The cumulative HBsAg seroclearance rate was 8% at 10 years, increased disproportionately to 25% at 20 years, and 45% at 25 years of follow-up [9]. HBsAg seroclearance usually confers excellent prognosis [10] .
Terminology and Natural History of Chronic HBV Infection
The consensus definition and diagnostic criteria for clinical terms relating to HBV infection adopted at the National Institutes of Health (NIH) conferences on Management of Hepatitis B in 2000 and 2006 are summarized in Table 1[11, 12]
Table 1- Definitions related to Chronic Hepatitis B
-
Chronic hepatitis B— Chronic necroinflammatory disease of the liver caused by persistent infection with hepatitis B virus. Chronic hepatitis B can be subdivided into HBeAg positive and HBeAg negative chronic hepatitis B.
-
Inactive HBsAg carrier state— Persistent HBV infection of the liver without significant, ongoing necroinflammatory disease.
-
Resolved hepatitis B— Previous HBV infection without further virologic, biochemical or histological evidence of active virus infection or disease.
-
Acute exacerbation or flare of hepatitis B— Intermittent elevations of aminotransferase activity to more than 10 times the upper limit of normal and more than twice the baseline value.
-
Reactivation of hepatitis B— Reappearance of active necroinflammatory disease of the liver in a person known to have the inactive HBsAg carrier state or resolved hepatitis B.
Evaluation and Management of Patients with Chronic HBV Infection
Initial Evaluation- Detailed history and physical examination forms part of every management protocol. In case of HBV, special emphasis is paid on risk factors for co-infection, alcohol use, and family history of HBV infection and liver cancer. Laboratory tests should include assessment of liver disease, markers of HBV replication, and tests for co-infection with HCV, HDV or HIV in those at risk (Table 2).
Table 2- Evaluation of patients with chronic hepatitis B infection
Initial evaluation-
- History & physical examination
- Family history of liver disease
- Laboratory tests to assess liver disease- complete blood counts with platelets, liver functions and prothrombin time
- Tests for HBV replication- HBeAg/anti-HBe, HBV DNA quantification
- Ultrasound of liver/rule out HCV, HDV and alcohol
- Tests to screen for hepatocellular carcinoma (serum alpha-fetoprotein)
- Consider liver biopsy to grade and stage liver disease for patients who meet criteria for chronicity.
Once patient is found to be HBsAg positive then the next step is to find the replicative status and the status of the liver injury in the liver by the virus.
STEP 1- The replicative status is diagnosed with the help of getting the serological marker that is HBeAg and Anti-HBeAg. The HBeAg is marker for the replication. With the increasing prevalence of the HBeAg negative chronic hepatitis B in which the virus multiply in the liver but do not produce the HBeAg antigen due to mutation the role of HBeAg as the marker of replicative stage has taken a back-seat. Nowadays most clinicians get the HBV DNA quantitative serum levels to know the replicative state of the virus. The levels of the HBV DNA do not depend on the HBeAg status and also helps in following the patients on therapy
STEP 2- After knowing the replicative status the next important step is to know the injury in the liver. There are two components of the damage that is fibrosis and inflammation. First is the fibrosis in the liver which occurs due to the long term inflammation leading to the activation of the hepatic stellate cells. This can only be estimated with the help of the liver biopsy though the role of fibroscan for same is coming up in the literature but still has not been placed in the guidelines. Next important step is to know the inflammation in the liver which can be either judged in the liver biopsy or indirectly by the AST/ALT levels. The importance of the ALT levels also lies in the fact that indication for the starting the treatment depends on the ALT levels which usually must be >1.5-2 times the Upper limit of the normal.
STEP 3-Third step is to know the status of the liver disease that is whether it is the state of hepatitis or state of chronic liver disease. The features that suggests the chronic liver disease are: shrunken liver, ascites, dilated portal vein, splenomegaly, oesophageal varices. The presence of these features changes the choice of the treatment protocol.
Liver Biopsy
The purpose of a liver biopsy is to assess the degree of liver damage and to rule out other causes of liver disease. As per the latest guidelines from the various groups the liver biopsy is not usually required in the treatment decision except in the condition where there is no clear cut guidelines for the treatment for example in patient with HBsAg positive with the ALT levels in the range of 1-2 times upper limit of normal in a person above 40 years [13].
HBV DNA Assays
As has been discussed above, HBV DNA quantification in serum is a crucial component in the evaluation of patients with chronic HBV infection and in the assessment of the efficacy of antiviral treatment. As HBV DNA persists even in persons who have serological recovery from acute hepatits B infection, low levels of HBV DNA may not be associated with progressive liver disease. An arbitrary value of 20,000 IU/mL was chosen as a diagnostic criterion for chronic hepatitis B at the 2000 NIH Conference [11]. It has been observed that serial monitoring of HBV DNA levels is more important than any single arbitrary cutoff value in prognostication and in determining the need for treatment. It is now recognized that lower HBV DNA levels (3-5 log10 IU/mL) may be associated with progressive liver disease and may warrant treatment, particularly in those who are HBeAg-negative or have already developed cirrhosis.
Treatment of Chronic Hepatitis B
Every patient with chronic HBV infection is potentially infectious and at risk for liver complications and is ideally a candidate for therapy, if the virus can be eradicated [14]. However, current medications rarely achieve viral eradication in patients with chronic HBV infection and therefore only patients who are at risk for progression to advanced liver disease should be considered for treatment.
Chronic hepatitis B is a chronic disease which is slowly progressive with dreaded results in the form of chronic liver disease and hepatocellular carcinoma (HCC). Thus aims of treatment are to achieve sustained suppression of HBV replication and remission of liver disease in order to prevent cirrhosis, hepatic failure and HCC.
Indication of treatment in patients with chronic hepatitis B are: ALT > 1.5 times the upper limit of normal, no evidence of the chronic liver disease as outlined above, DNA more than 20,000 IU/mL and 2000 IU/mL in HBeAg positive and HBeAg negative patients, respectively [15,16]. Treatment algorithms for chronic HBV infection is outlined in Figures 1 and 2.
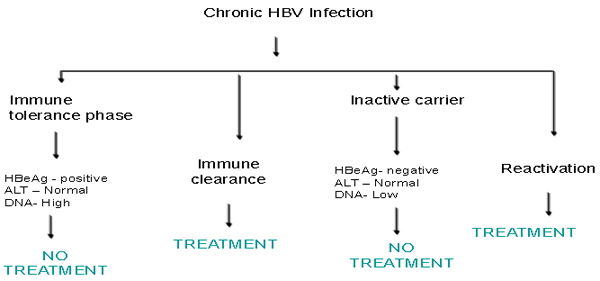
Figure 1- Outline of management of chronic hepatitis B virus infection
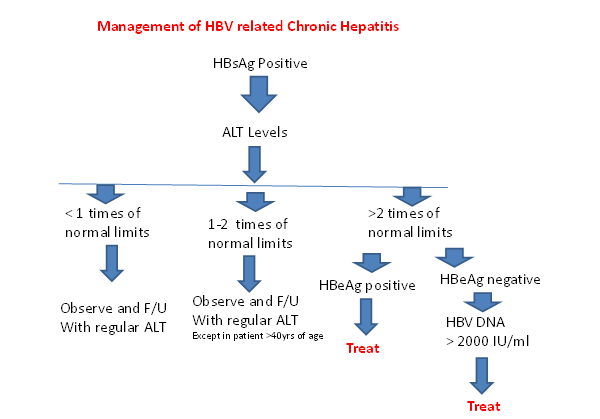
Figure 2- Management of HBV related chronic hepatitis
Currently, there are seven drugs licensed for treatment of Chronic Hepatitis B: standard IFN-alpha, pegylated IFNα-2a (Peg- IFNalpha-2a) (Peg-IFNα-2b is also licensed in some countries), lamivudine (LAM), adefovir dipivoxil (ADV), entecavir (ETV), telbivudine (TBV) and tenofovir disoproxil fumarate (TDF). Thus, two different classes of drugs are available for treatment- interferon therapy and oral nucleoside/tide therapy. The merits and drawbacks of these two are outlined in Table 3.
Table 3- Advantages and Drawbacks of drugs available for treatment
|
Advantages |
Drawbacks |
Interferon |
Finite treatment
No resistance
Loss of HBsAg
Durable off-treatment response |
Injectable
High rate of side effects
High Cost
Low response with high levels of HBV DNA |
Oral Nucleoside |
Oral drugs
Negligible side effects
Low price
Potent inhibition of viral replication |
No finite treatment
Resistance to drugs |
Table 4- Management of chronic hepatitis B according to different factors
HBeAg |
HBV DNA |
ALT |
Management |
+ |
>20,000 IU/mL |
< 2 x ULN |
Observe |
+ |
>20,000 IU/mL |
> 2 x ULN |
Treat |
- |
≤ 2000 IU/mL |
≤ 2 ULN |
Observe |
- |
>2000 IU/mL |
1-2 ULN |
Consider Biopsy |
- |
>2000 IU/mL |
> 2 x ULN |
Treat |
+/- (in cirrhosis) |
Positive |
Any value |
Treat |
+/- (in cirrhosis) |
Undetectable |
Any value |
Compensated cirrhosis- Observe
Decompensated cirrhosis- Liver transplantation |
Interferon-alfa
Interferons (IFNs) have antiviral, antiproliferative, and immunomodulatory effects. IFN has been shown to be effective in suppressing HBV replication and in inducing remission of liver disease.
IFN are of two types: Conventional and Pegylated IFN.IFN is administered as subcutaneous injections. The recommended dose for conventional IFN in adults is 5 MU daily or 10 MU thrice weekly and for children 6 MU/m2 thrice weekly with a maximum of 10 MU. The recommended duration of treatment for patients with HBeAg positive chronic hepatitis B is 16 to 24 weeks. Current data suggest that patients with HBeAg-negative chronic hepatitis B should be treated for at least 12 months. Pegylated interferon has been found to be more effective than conventional interferon in the treatment of HBV infection. Doses of 1.0 μg/kg body weight of pegylated interferon alfa-2b and 180 μg of pegylated interferon alfa-2a are given once weekly.
IFN induced HBeAg clearance has been reported to be durable in 80% to 90% of patients after a follow-up period of 4 to 8 years. In a study comparing the outcome of treated patients and controls from Taiwan, an 8-year follow-up of 101 male patients who participated in a controlled trial of IFN therapy found that treated patients had a lower incidence of HCC (1.5% vs 12%, p= 0.04) and a higher survival rate (98% vs 57%, p=0.02) [17]. But these good results are not seen in the HBeAg-negative patients, in whom the relapse after cessation of IFN treatment is frequent with sustained response rates of only 15% to 30%.
Table 5- Drug dosage and duration for chronic hepatitis B therapy.
Drugs – Dose & Duration
|
Dose |
Duration
HBeAg positive |
Duration
HBeAg positive |
IFN alpha |
5 MU /day
10 MU thrice weekly |
16 – 24 wks |
48wks |
Peg IFN |
1.5ug/kg/day
180ug/kg/day |
48wks |
> 48wks |
Entecavir |
0.5mg OD
1 mg OD |
> 1 yrs |
> 2 yrs |
Tenofovir |
300 mg OD |
> 1 yrs |
> 2 yrs |
Lamivudine |
100 mg OD |
> 1 yrs |
> 2 yrs |
Nucleoside analogues
Nucleoside analogues replace natural nucleosides during the synthesis of the first or second strand (or both) of HBV DNA. They thus serve as competitive inhibitors of the viral reverse transcriptase and DNA polymerase. Nucleoside analogues have excellent oral bioavailability, a good safety record and antiviral efficacy comparable to that observed with interferon alfa-2b. They also are considerably less expensive than interferon when given for 48 weeks. One of the important drawbacks of the oral nucleoside analogues is the development of the resistance (table 6).
Lamivudine
Lamivudine has been shown to be a relatively potent inhibitor of viral replication, convenient to administer and free of severe adverse effects. Clinical trials demonstrated that a 1-year course of lamivudine resulted in suppression of viral replication and improvement in histologic findings in the liver [18]. In one study, HBeAg seroconversion and HBeAg loss occurred in 17% and 32% of patients, respectively [19]. Prolongation of treatment beyond 1 year, however, has been associated with incremental changes in viral resistance (38% at 2 years), and the longer treatment is continued, the more frequently resistance is seen (65% at year 5) [20]. In an Indian study, the annual incremental loss of HBeAg in patients receiving lamivudine was 41.6% at the end of 1st year, 55% at 2nd year and 58.3% at 3rd year [21].
Adefovir Dipivoxil
Adefovir dipivoxil is the acyclic phosphonate nucleotide analogue of adenosine mono-phosphate. The rates of HBeAg seroconversion and HBeAg loss were slightly lower than those achieved with lamivudine for 52 weeks (12% and 24%, respectively). Point mutations (A181V, N236T) of the HBV polymerase gene affect HBV susceptibility to adefovir but occur in 18% of patients at 4 years and 29% at 5 years. In comparison, lamivudine resistance is approximately 15 to 20 times as common at the same time intervals. Adefovir is clinically and virologically effective in patients with lamivudine-resistant HBV, whether they have clinically stable disease, decompensated cirrhosis, or recurrent hepatitis B after liver transplantation. Adefovir has the disadvantage of being potentially nephrotoxic, and dose reductions may be necessary in patients likely to experience compromised renal function.
Tenofovir Disoproxil Fumarate
Tenofovir, an acyclic nucleotide inhibitor of HBV polymerase and HIV reverse transcriptase, is similar chemically to adefovir dipivoxil. Tenofovir has been licensed for the treatment of HIV infection, and its antiviral activity against HBV has been reported to be greater than that of the 10-mg dose of adefovir in lamivudine-resistant patients. More than 90% of HBeAg-negative patients and nearly 80% of HBeAg-positive patients treated with tenofovir have persistent virologic responses and HBV DNA levels less than 400 copies/mL by 72 weeks, with minimal side effects. Marcellin et al reported no development of resistance to tenofovir after 48 weeks of treatment. Although the nucleotide analogues have been associated with renal toxicity, the risk of renal toxicity associated with tenofovir is 1% or less per year; it can be reduced even further by calculating renal function through the use of the Cockroft-Gault equation or Modification of Diet in Renal Disease equation prior to therapy and adjusting the dosage accordingly. With profound HBV DNA suppression, HBsAg loss occurs in about 5% of tenofovir-treated patients at 64 weeks. Treatment with tenofovir in treatment-experienced patients leads to potent suppression of HBV DNA independent of HBV genotype, HBV mutations (YMDD mutations) that signal lamivudine resistance, or HBeAg status at baseline. Patients with genotypic resistance to adefovir at baseline had a lower probability of achieving HBV DNA suppression during treatment with tenofovir [22].
Entecavir
Entecavir induces profound suppression of HBV DNA (to undetectable levels by weeks 24 to 36) in patients who are HBeAg positive or negative, regardless of baseline HBV DNA levels; resistance rates are very low in treatment-naive patients, and entecavir is therefore considered first-line therapy [23]. More than 90% of HBeAg-positive or -negative patients who are adherent to entecavir are HBV DNA negative at 5 years. Loss of HBsAg is 5% in entecavir-treated patients at follow-up of approximately 80 weeks, which is roughly double the rate of HBsAg loss with lamivudine [24]. Entecavir is effective against both wild type and lamivudine resistant HBV. It is more potent than either lamivudine or adefovir.
Cumulative Annual Incidence of Resistance
Drug |
1st yr |
2nd yr |
3rd yr |
4th yr |
5th yr |
6th yr |
Lamivudine |
23 |
46 |
55 |
71 |
80 |
- |
Adefovir |
0 |
3 |
6 |
18 |
29 |
- |
Tenfovir |
0 |
0 |
- |
- |
- |
- |
Entecavir |
0.2 |
0.5 |
1.2 |
1.2 |
1.2 |
1.2 |
Table 6- Resistance pattern of different oral antiviral drugs against HBV
Antiviral Therapy in Special Populations
Antiviral therapy can be considered during pregnancy to protect the health of the mother and to prevent breakthrough HBV infection in HBV-vaccinated newborns. However, none of the current antiviral agents is licensed for use in pregnancy. Extensive experience with tenofovir exists in HIV-HBV co-infected mothers. Lamivudine is a category B drug in HIV-infected pregnant women but a category C drug in HBV-infected women. Tenofovir is also considered as category B drug in HBV-infected mothers. Because lamivudine has a long record of safety and has had the most extensive use during pregnancy in HIV-infected women, many hepatologists prefer to prescribe this agent whenever they feel compelled to treat hepatitis B in a pregnant woman. Defects in bone mineral density, including osteomalacia, have been described with tenofovir in HIV-infected patients.
Interferon is contraindicated during pregnancy largely because of its antiproliferative effects. In the event of pregnancy, interferon should be discontinued. Breast feeding is not recommended during the first year of the infant's life for mothers who are undergoing antiviral therapy.
during the first year of the infant's life for mothers who are undergoing antiviral therapy.
1. Persons with Acute Hepatitis
Because of the high rate (>95%) of complete immunologic recovery from acute hepatitis B, definitive recommendations about the treatment of acute hepatitis B cannot be made. Some experts recommend nucleoside analogue therapy when HBeAg remains detectable in serum for more than 10 to 12 weeks. A National Institutes of Health–sponsored clinical workshop on hepatitis B proposed that persons with acute viral hepatitis complicated by an increase in INR above 1.5 and deep jaundice persisting for more than four weeks should receive antiviral therapy [25].
2. Persons with Cirrhosis
Nucleoside analogue therapy has been shown to be safe in patients with cirrhosis. Interferon is contraindicated in patients with even mildly decompensated cirrhosis because immune-mediated flares of serum ALT levels may occur. Practice guidelines of the AASLD suggest that nucleoside analogue therapy is preferred in all cases of HBV-related cirrhosis.
3. Persons with Human Immunodeficiency Virus & Hepatitis B Virus Co-infection
Antiviral therapy for hepatitis B should be considered for all HIV-HBV co-infected patients with evidence of liver disease, irrespective of the CD4 count. Choice of treatment varies with stage of HIV infection. Adefovir and pegylated interferon may be employed for patients not on anti-retroviral therapy while those on anti-retroviral therapy can be started on tenofovir along with either of emtricitabine or lamivudine.
4. Persons with Hepatitis B Virus–Hepatitis C Virus Co-infection
HBV-HCV co-infected patients tend to have more severe liver injury and a higher probability of cirrhosis. Usually, one virus, often HCV, is dominant through the process of viral interference. The typical patient is positive for HCV RNA but negative for HBV DNA in serum. Close monitoring has been recommended before treatment is initiated, however, because some patients exhibit alternating viremia, the optimal therapy for patients is unclear. Different combinations of a nucleoside analogue, pegylated interferon alpha and ribavirin are being tried.
Conclusion
The decision to treat any patient with chronic HBV infection should be based on reasonable clinical judgment. Therapeutic strategies for chronic hepatitis B can be summarized as therapies of finite duration aiming to offer sustained off-therapy response and long-term therapies aiming to maintain remission under oral antiviral agents. Due to high rate of resistance against the oral antiviral drugs judicious use of oral anti-HBV agents is recommended, particularly in patients with mild liver disease. Regardless of the anti-HBV agent used, compliance should always be ascertained and most current guidelines recommend HBV DNA testing at least every 6 months for the prompt diagnosis of lack of response or virological breakthroughs and timely treatment modification.
References
-
Prevention of Hepatitis B in India- An Overview. World Health Organization South-East Asia Regional office, New Delhi; 2002. whqlibdoc.who.int/searo/
2002/SEA_Hepat.-5.pdf. Accessed on October 13, 2010.
-
Nayak NC, Panda SK, Zuckerman AJ, Bhan MK, Guha DK. Dynamics and impact of perinatal transmission of hepatitis B virus in North India. J Med Virol 1987;21:137-45.
-
Chakravarty R, Chowdhury A, Chaudhuri S, Santra A, Neogi M, Raendran K, Panda CK, Chakravarty M. Hepatitis B infection in Eastern Indian families: Need for screening of adult siblings and mothers of adult index cases. Public Health 2005;119:647-54.
-
Liaw YF. Hepatitis flares and hepatitis B e antigen seroconversion: implication in anti-hepatitis B virus therapy. J Gastroenterol Hepatol 2003;18:246-52.
-
Chu CM, Liaw YF. Genotype C hepatitis B virus infection is associated with a higher risk of reactivation of hepatitis B and progression to cirrhosis than genotype B: a longitudinal study of hepatitis B e antigen-positive patients with normal aminotransferase levels at baseline. J Hepatol 2005;43:411–7.
-
Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med 2004;116:829-34.
-
Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology 2002;35:1522–7.
-
Chu CM, Liaw YF. Predictive factors for reactivation of hepatitis B following hepatitis B e antigen seroconversion in chronic hepatitis B. Gastroenterology 2007;133:1458–65.
-
Chu CM, Liaw YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: appreciably high rates during a long-term follow up. Hepatology 2007;45:1187–92.
-
Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology 2002;123:1084–9.
-
Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000-summary of a workshop. Gastroenterology 2001;120:1828-53.
-
Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology 2007; 45:1056-75.
-
Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50:661-2.
-
Papatheodoridis GV, Manolakopoulos S, Dusheiko G, Archimandritis AJ. Therapeutic strategies in the management of patients with chronic hepatitis B virus infection. Lancet Infect Dis 2008;8:167-78.
-
Liaw YF, Leung N, Guan R, Lau GK, Merican I, McCaughan G, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005update. Liver Int 2005;25:472-89.
-
Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: an update. Clin Gastroenterol Hepatol 2006;4:936-62.
-
Lin SM, Sheen IS, Chien RN, Chu CM, Liaw YF. Long-term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology 1999;29:971-5.
-
Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, et al: A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study group. N Engl J Med 1998; 339:61-8.
-
Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med 1999;341:1256-63.
-
Lok ASF, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, et al: Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003; 125:1714-22.
-
Alexander G, Baba CS, Chetri K, Negi TS, Choudhuri G. High rates of early HBeAg seroconversion and relapse in Indian patients of chronic hepatitis B treated with Lamivudine: results of an open labeled trial. BMC Gastroenterol 2005;5:29.
-
Gish RG. Hepatitis B treatment: current best practices, avoiding resistance. Cleve Clin J Med 2009;76 suppl 3:S14-9.
-
Colonno RJ, Rose R, Baldick CJ, Levine S, Pokornowski K, Yu CF, et al.. Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology 2006; 44:1656–65.
-
Perrillo RP. Current treatment of chronic hepatitis B: benefits and limitations. Semin Liver Dis 2005;25 suppl 1:20–8.
-
Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology 2007;45:1056-75.


